The intestines of an adult host a large number of different bacterial species. These are mainly present in the lower part of the gastrointestinal (GI) tract and form a symbiotic relationship with their human home. Several current studies describe the way the gut microbiota communicates with the skin as one of the main regulators of the gut-skin axis. The main focus of this research is to explore its potential influence on skin differentiation and cellular hornification, the modulation of the cutaneous immune response in various diseases and how this communication can be utilised to control various skin conditions.
Gut and skin, both densely vascularised and richly innervated organs with crucial immunological and neuroendocrine roles, are uniquely related in terms of purpose and function (1). As our primary gateway to the external environment, both organs are essential for maintaining physiological homeostasis. Several studies have demonstrated a close, bidirectional link between the gut and our skin. Additionally, numerous studies have linked GI health to skin homeostasis and adjustment mechanisms (1,2).
Gastrointestinal disorders are often accompanied by cutaneous manifestations such as blemishes and/or rashes. Moreover, the GI system, particularly the gut microbiota, appears to be involved in the pathophysiology of many inflammatory diseases (3,4,5). The skin diseases most commonly associated with a disturbed gut microbiota are acne, atopic dermatitis and psoriasis (Figure 1).
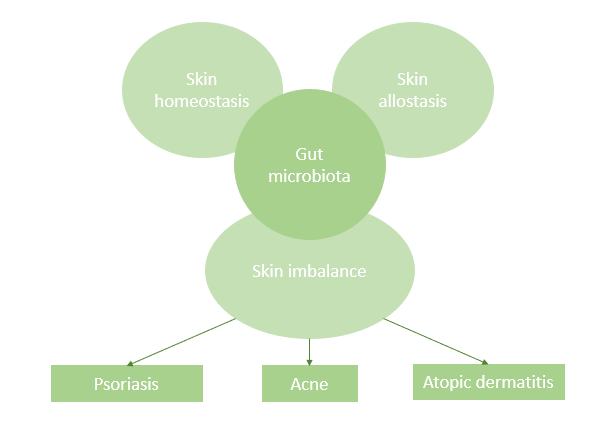
Figure 1: The gut-skin axis: Connection between the skin, intestine and skin diseases
The influence of the gut microbiota on skin homeostasis
The skin fulfils three main functions: protection from external influences, temperature regulation and water storage. However, it can only perform these and more activities in a state of homeostasis (balance). To maintain this state, an effective epidermal process — through which the skin regenerates itself — is essential. Epidermal cells arise from stem cells in the basal layer of the epidermis and undergo morphological changes as they reach the surface of the skin.
The cells differentiate into three types (basal, spiny and granule) before eventually becoming the corneocytes that form the outermost layer of the epidermis. This process of epidermal differentiation, also known as hornification (keratinisation), is controlled by specific transcriptional pathways (6,7, 8, 9).
Ultimately, this highly regulated process leads to the production of the outermost skin layer, the stratum corneum, which consists of about 15 layers of densely keratinised corneocytes held together by multiple lipid bilayers in a “bricks and mortar” model. The corneocytes serve as bricks, whereas ceramides, cholesterol, fatty acids and cholesterol esters form the mortar that holds the bricks together.
When epidermal regeneration functions appropriately, the resulting bricks-and-mortar structure serves as an effective barrier with the ability to limit evaporation, retain moisture and protect the skin from invasion by foreign organisms and substances (6, 7, 8). By way of its influence on the signalling pathways that coordinate this process, which is important for skin homeostasis, the intestinal microbiota influences skin health (1).
The intestinal microbiota also contributes to the skin’s adaptation process and the restoration of homeostasis after a disruption or stressor via gut-mediated effects on innate and adaptive immunity (10, 11, 12). Studies have shown that gut bacteria can positively influence the body’s response to impaired skin barrier function. For example, Baba et al. (2010) demonstrated that the administration of Lactobacillus helveticus can reduce the severity of sodium dodecyl sulphate-induced dermatitis. Another study described how recovery of the skin barrier function was improved.
Intestinal dysbiosis and skin diseases
Intestinal dysbiosis, a state of microbial imbalance in the gut, has the potential to negatively affect skin function. Free phenol and p-cresol, metabolites of aromatic amino acids, are used as biomarkers of a disturbed intestinal environment because their production is induced by certain pathogenic bacteria, especially Clostridium difficile. These metabolites are able to influence the metabolic status quo, preferentially accumulating in the skin and affecting epidermal differentiation and skin barrier integrity (1). Particularly high serum p-cresol levels are associated with reduced skin hydration and impaired hornification (13, 14).
Intestinal dysbiosis leads to increased epithelial permeability, which then triggers the activation of effector T cells and destroys their relationship with immunosuppressive regulatory T cells. Pro-inflammatory cytokines further increase epithelial permeability and initiate a vicious cycle of chronic systemic inflammation (1, 15). These are just some of the mechanisms by which a disturbed gut microbiota manifests itself in impaired skin function.
Treatment and prevention of intestinal dysbiosis
The gut microbiota is strongly influenced by diet. Although long-term dietary habits shape bacterial composition, a dramatic change in diet during a short period of time can quickly produce other undesirable “gut inhabitants”. Given the influence of the gut microbiota on the development of inflammatory diseases, this makes it possible to modify the microbiota in a targeted manner using therapeutic agents such as live beneficial gut bacteria or “probiotics” (16). The use of probiotics has already shown promising results in the prevention and treatment of various diseases (17, 18, 19, 20, 21, 22).
The use of probiotics has been particularly successful in the treatment of acne. Certain strains can suppress the bacterium, Propionibacterium acnes, which is responsible for acne outbreaks, among other factors. By secreting an antibacterial protein, the growth of this bacterium is inhibited (25).
Of course, many strains of probiotic bacteria exist, but the most important ones to consider in the context of skin health are Lactobacillus species and Bifidobacterium. In a recent human study, a significant decrease in water loss through the skin epidermis and increased skin hydration was observed after 12 weeks of oral supplementation with Lactobacillus brevis (9). A different study also showed a significant improvement in skin elasticity and increased skin hydration after 12 weeks of oral supplementation with Lactobacillus plantarum (25).
Nutrition is widely recognised as a key factor in terms of mediating the function of the gastrointestinal microbiome. Dietary fibre undergoes a process of bacterial fermentation in the GI tract, producing short-chain fatty acids (SCFAs) that promote a healthy colon. SCFAs can promote the growth of certain skin microbes that influence immune defence and regulate the skin’s inflammatory potential (25). Studies have also shown that higher dietary fibre intake is associated with increased gut microbial diversity; furthermore, a diverse population of bacterial strains in the gut is critical for gut and, therefore, skin health (25).
Author: Laura Ingenlath, Quality Manager, Taiyo GmbH.
This article “The gut microbiota as a regulator of the gut-skin axis” is a translation of the article: “Die Darm-Mikrobiota als Regler der Haut-Darm-Achse”, published in the German magazine “Vitalstoffe 2/2021”, BK nutri network.
Do you have any questions regarding the gut-skin axis and Taiyo’s dietary fiber Sunfiber® for an increased gut microbial diversity? Please do not hesitate to contact us!
References:
- O’Neill, C. A., Monteleone, G., McLaughlin, J. T., and Paus, R. (2016). The gut-skin axis in health and disease: a paradigm with therapeutic implications. Bioessays 38, 1167–1176. doi: 10.1002/bies.201600008
- Levkovich, T., Poutahidis, T., Smillie, C., Varian, B. J., Ibrahim, Y. M., Lakritz, J. R., et al. (2013). Probiotic bacteria induce a ‘glow of health’. PLoS One 8:e53867. doi: 10.1371/journal.pone.0053867
- Shah, K. R., Boland, C. R., Patel, M., Thrash, B., and Menter, A. (2013). Cutaneous manifestations of gastrointestinal disease: part I. JAAD 68, 189.e1-189.e21. doi: 10.1016/j.jaad.2012.10.037
- Thrash, B., Patel, M., Shah, K. R., Boland, C. R., and Menter, A. (2013). Cutaneous manifestations of gastrointestinal disease: part II. JAAD 68, 211.e1-211.e33. doi: 10.1016/j.jaad.2012.10.036
- Gloster, H. M., Gebauer, L. E., and Mistur, R. L. (2016). “Cutaneous manifestations of gastrointestinal disease,” in Absolute Dermatology Review, eds H. M. Gloster, L. E. Gebauer, and R. L. Mistur (Cham: springer), doi: 10.1007/978-3-319-03218-4_48
- Baba, H., Masuyama, A., and Yoshimura, C. (2012). Promoter of differentiation and keratinization of epidermic cell and functional beverage/food for promotion of keratinization of epidermis. U.S. Patent NO CA2614111A1
- Weaver, C. T., Elson, C. O., Fouser, L. A., and Kolls, J. K. (2013). The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 24, 477–512. doi: 10.1146/annurev-pathol-011110-130318
- Gaur, M., Dobke, M., and Lunyak, V. V. (2014). Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int. J. Mol. Sci. 18:208. doi: 10.3390/ijms18010208
- Abhishek, S., Tech, M., and Krishnan, S. P. (2016). Epidermal differentiation complex: a review on its epigenetic regulation and potential drug targets. Cell J. 18, 1–6. doi: 10.22074/cellj.2016.3980
- Benyacoub, J., Bosco, N., Blanchard, C., Demont, A., Philippe, D., CastielHigounenc, I., et al. (2014). Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences. Benef. Microbes 5, 129–136. doi: 10.3920/BM2013.0014
- Kim, H. M., Lee, D. E., Park, S. D., Kim, Y., Kim, Y. J., Jeong, J. W., et al. (2014). Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 24, 1583–1591. Doi:10.4014/jmb.1406.06038
- Chen, Y., Wu, C., Chao, Y., Lin, C., Tsai, H., Li, Y., et al. (2017). Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J. Food Drug Anal. 25, 559–566. doi: 10.1016/j.jfda.2016. 06.003
- Dawson, L. F., Donahue, E. H., Cartman, S. T., Barton, R. H., Bundy, J., McNerney, R., et al. (2011). The analysis of para-cresol production and tolerance in Clostridium difficile 027 and 012 strains. BMC Microbiol. 11:86. doi: 10.1186/1471-2180-11-86
- Miyazaki, K., Masuoka, N., Kano, M., and Iizuka, R. (2014). Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef. Microbes 5, 121–128. doi: 10.3920/BM2012.0066
- Kosiewicz, M. M., Dryden, G. W., Chhabra, A., and Alard, P. (2014). Relationship between gut microbiota and development of T cell associated disease. FEBS Lett. 588, 4195–4206. doi: 10.1016/j.febslet.2014.03.019
- Huang, Y. J., Marsland, B. J., Bunyavanich, S., O’Mahoney, L., Leung, D. Y. M., Muraro, A., et al. (2017). The microbiome in allergic disease: current understanding and future opportunities – 2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 139, 1099–1110. Doi: 10.1016/j.jaci.2017.02.007
- Krutmann, J. (2009). Pre-and probiotics for human skin. J. Dermatol. Sci. 54, 1–5. doi: 10.1016/j.jdermsci.2009.01.002
- Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
- Farris, P. K. (2016). Are skincare Products with probiotics worth the hype? Dermatology Times, 8th, August
- Grant, M. C., and Baker, J. S. (2017). An overview of the effect of probiotics and exercise on mood and associated health conditions. Crit. Rev. Food Sci. Nutr. 57, 3887–3893. Doi: 10.1080/10408398.2016.1189872Sánchez
et al., 2017
- Sarao, L. K., and Arora, M. (2017). Probiotics, prebiotics, and microencapsulation: a review. Crit. Rev. Food Sci. Nutr. 57, 344–371. doi: 10.1080/10408398.2014
. 887055
- Muizzuddin, N., Maher, W., Sullivan, M., Schnittger, S., and Mammone, T. (2012). Physiological effect of a probiotic on skin. J. Cosmet. Sci. 63, 385–395
- Frei, R., Akdis, M., and O’Mahony, L. (2015). Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr. Opin. Gastroenterol. 31, 153–158. doi: 10.1097/MOG.0000000000000151
- Salem, I. Ramser, A. Ghannoum, MA. (2018). ‘The Gut Microbiome as a Major Regulator of the Gut-Skin Axis’, Frontiers in Microbiology, (9), pp.1459. Last access 31 May 2021. Accessible at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6048199/
#!po=2.77778
- Holscher, HD. (2017). ‘Dietary fiber and prebiotics and the gastrointestinal microbiota’, Gut Microbes, 8 (2), pp. 172-184. Last access 31 May 2021. Accessible at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5390821/
Picture source: Shutterstock | Rido






